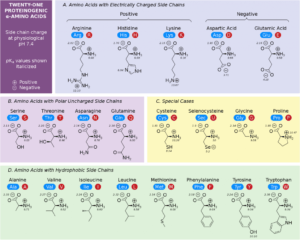
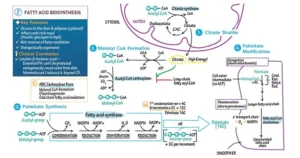
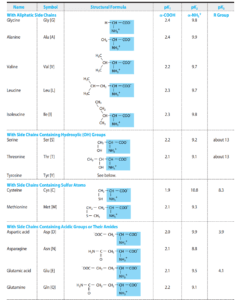
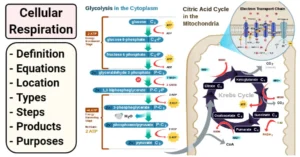
Biomedical Importance
Proteins are the workhorses of the cell. Their biological function depends not just on their amino acid sequence (primary structure) but on how they fold into higher-order structures. Misfolding or defects in stabilization underlie many diseases, including Alzheimer’s, cystic fibrosis, and prion disorders. Understanding higher-order structure is therefore essential for drug design, biomaterials, and molecular diagnostics.
Conformation Versus Configuration
- Configuration refers to the fixed arrangement of atoms in a molecule that can only be changed by breaking covalent bonds (e.g., L- vs. D-amino acids).
- Conformation refers to the spatial arrangement of atoms that can change by rotation about single bonds without breaking covalent bonds (e.g., different folds of a polypeptide).
Proteins exhibit dynamic conformations but retain a fixed configuration.
Proteins Were Initially Classified by Their Gross Characteristics
Early biochemists classified proteins as fibrous (e.g., collagen, keratin) or globular (e.g., enzymes, hemoglobin) based on solubility, shape, and role. This gross classification laid the groundwork for understanding structure–function relationships.
Proteins Are Constructed Using Modular Principles
Most proteins are built from domains—compact, independently folding units that combine to create multidomain proteins. These domains often correspond to functional modules (binding, catalysis, regulation), reflecting the modular nature of evolution.
The Four Orders of Protein Structure
Secondary Structure
Regular, recurring structures stabilized by hydrogen bonds between backbone groups.
- Peptide Bonds Restrict Possible Secondary Conformations:
The partial double-bond character of the peptide bond makes it planar and limits rotation. Only the angles around the α-carbon (ϕ and ψ) vary, as seen in Ramachandran plots. - The Alpha Helix:
A right-handed coil with 3.6 residues per turn. Hydrogen bonds form between the carbonyl oxygen of residue i and the amide hydrogen of residue i+4. Common in many globular proteins.

- The Beta Sheet:
Polypeptide strands align side-by-side. Hydrogen bonds form between adjacent strands in either parallel or antiparallel orientation, producing pleated sheets.

- Loops & Bends:
Irregular segments connecting α-helices and β-sheets. They provide flexibility, surface exposure, and sites for interactions.

Tertiary & Quaternary Structure
- Tertiary Structure is the complete 3-D fold of a single polypeptide chain, integrating α-helices, β-sheets, and loops into a functional domain.
- Quaternary Structure arises when two or more polypeptide chains (subunits) assemble into a functional protein complex, as in hemoglobin.

Multiple Factors Stabilize Tertiary & Quaternary Structure
Stability is conferred by a combination of hydrophobic interactions, hydrogen bonds, ionic interactions, van der Waals forces, and covalent disulfide bonds. These forces act synergistically to maintain native conformation.
Three-Dimensional Structure Is Determined by X-Ray Crystallography or by NMR Spectroscopy
- X-Ray Crystallography:
High-resolution technique for determining the atomic structure of crystallized proteins. It has provided most known protein structures. - Nuclear Magnetic Resonance (NMR) Spectroscopy:
Allows structure determination of proteins in solution, revealing dynamics as well as structure for smaller proteins. - Molecular Modeling:
Computational tools predict structures from sequence data or refine experimental models, useful when experimental data are limited.
Protein Folding
- The Native Conformation of a Protein Is Thermodynamically Favored:
Folding is a spontaneous process driven by the decrease in free energy as hydrophobic residues bury inside and stabilizing interactions form. - Folding Is Modular:
Domains often fold independently, then assemble into larger structures. - Auxiliary Proteins Assist Folding:
Cells use helper proteins to ensure proper folding:- Chaperones prevent aggregation and provide a protected environment for folding.
- Protein Disulfide Isomerase (PDI) catalyzes the reshuffling of disulfide bonds.
- Proline-cis/trans-Isomerase accelerates the slow isomerization of proline residues.

Several Neurologic Diseases Result from Altered Protein Conformation
- Prions: Infectious proteins that propagate by inducing misfolding of normal cellular proteins, causing diseases like Creutzfeldt–Jakob disease.
- Alzheimer’s Disease: Characterized by misfolded amyloid-β peptides forming insoluble plaques and tau tangles, leading to neuronal death.
These examples underscore the health impact of protein misfolding.
Collagen Illustrates the Role of Posttranslational Processing in Protein Maturation
- Protein Maturation Often Involves Making & Breaking Covalent Bonds:
Hydroxylation, glycosylation, cleavage of precursors, and cross-linking stabilize mature proteins. - Collagen Is a Fibrous Protein:
Provides structural strength to connective tissues. - Collagen Forms a Unique Triple Helix:
Three left-handed polypeptide chains wrap around each other to form a right-handed triple helix stabilized by hydrogen bonds. - Collagen Is Synthesized as a Larger Precursor:
Secreted as procollagen, then cleaved and cross-linked to form mature collagen fibers. - Nutritional & Genetic Disorders Can Impair Collagen Maturation:
Vitamin C deficiency (scurvy) impairs proline and lysine hydroxylation, weakening collagen. Genetic mutations cause disorders like osteogenesis imperfecta and Ehlers–Danlos syndrome.

Summary
- Biomedical Importance: Higher-order protein structures (secondary, tertiary, quaternary) determine biological function; misfolding underlies many diseases.
- Conformation vs. Configuration:
- Configuration = fixed arrangement requiring covalent bond changes (L- vs D-amino acids).
- Conformation = 3-D arrangement from rotations about single bonds; dynamic but reversible.
- Initial Classification: Early grouping into fibrous vs. globular proteins based on solubility and shape.
- Modular Construction: Proteins are built from independently folding domains that confer distinct functions.
- Four Orders of Structure:
- Primary: Amino acid sequence.
- Secondary: Local patterns—α-helices, β-sheets, loops.
- Tertiary: 3-D fold of one chain.
- Quaternary: Assembly of multiple subunits.
- Secondary Structure Details:
- Peptide bonds are planar and restrict rotation (ϕ, ψ angles).
- Alpha helix: Right-handed coil stabilized by H-bonds.
- Beta sheet: Parallel or antiparallel pleated strands.
- Loops & bends: Flexible connectors and interaction sites.
- Tertiary & Quaternary Structure Stabilization: Hydrophobic effect, hydrogen bonds, ionic interactions, van der Waals forces, and disulfide bonds.
- Structure Determination Methods:
- X-ray crystallography for high-resolution crystal structures.
- NMR spectroscopy for proteins in solution.
- Molecular modeling complements experimental data.
- Protein Folding:
- Native conformation is thermodynamically favored.
- Domains fold modularly.
- Chaperones, protein disulfide isomerase, and proline cis/trans-isomerase assist correct folding.
- Misfolding Diseases:
- Prions induce abnormal folding.
- Alzheimer’s disease involves amyloid-β and tau misfolding.
- Collagen as an Example of Posttranslational Processing:
- Fibrous protein forming a unique triple helix.
- Synthesized as procollagen, then cleaved and cross-linked.
- Requires vitamin C for hydroxylation; deficiencies or mutations impair maturation (e.g., scurvy, osteogenesis imperfecta).
- Key Concept: Protein function is inseparable from its higher-order structure; correct folding and posttranslational modifications are critical for stability and activity.