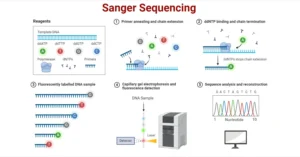
Illumina sequencing, often referred to as next-generation sequencing (NGS), is one of the most widely adopted methods for high-throughput DNA sequencing. It leverages sequencing by synthesis (SBS) technology to detect individual DNA bases as they are incorporated into a growing DNA strand. This method is faster, more accurate, and cost-effective compared to traditional techniques like Sanger sequencing, making it ideal for large-scale genomic and transcriptomic studies.

Principle of Illumina Sequencing
Illumina sequencing is based on the principle of sequencing by synthesis (SBS). The process involves incorporating nucleotides with unique fluorescent labels into a growing DNA strand. Each of the four nucleotides (A, T, C, G) is tagged with a specific fluorescent dye. As these nucleotides are added to the growing DNA strand, the system detects the emitted fluorescence, which corresponds to the identity of the nucleotide. This allows the system to identify which nucleotide has been added during each cycle.
To enhance the signal detection and improve the accuracy of sequencing, bridge amplification is used. DNA fragments are immobilized onto a solid surface (like a flow cell), and each fragment undergoes amplification to form clusters of identical DNA sequences. These clusters allow for the simultaneous sequencing of millions of DNA fragments in parallel, vastly increasing the throughput compared to older methods that could sequence only one DNA molecule at a time.
Steps/Process of Illumina Sequencing
The Illumina sequencing process can be divided into several critical steps:

- Nucleic Acid Extraction The first step in Illumina sequencing is the extraction of nucleic acids (DNA or RNA) from the sample. High-quality and well-quantified nucleic acids are crucial for generating accurate sequencing data. DNA extraction is typically performed using chemical or mechanical methods, depending on the type of sample. Quality control of the extracted DNA is performed using techniques like UV spectrophotometry or fluorometric assays to ensure the purity and concentration are suitable for downstream sequencing.
- Library Preparation After nucleic acid extraction, the next step is preparing the sample for sequencing by creating a DNA library. This library consists of DNA fragments that have been ligated with short adapter sequences at both ends. These adapters are essential for binding the DNA to the flow cell during sequencing. The process of library preparation includes:
- DNA fragmentation: The extracted DNA is fragmented into smaller pieces using methods such as mechanical shearing, enzymatic digestion, or transposon-based fragmentation.
- End repair and A-tailing: The fragmented DNA ends are repaired to create blunt ends, and a single adenine (A) base is added to the 3′ ends of the fragments.
- Adapter ligation: Short adapter sequences are ligated to both ends of the DNA fragments. These adapters contain sequences that are complementary to primers on the flow cell and often include a unique barcode for sample identification in multiplex sequencing.
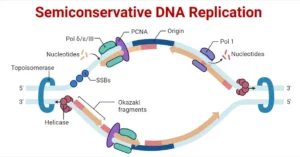
- Cluster Generation by Bridge Amplification Once the DNA library is prepared, it is loaded onto the flow cell, which is a small glass slide with lanes coated with oligonucleotides that are complementary to the adapters on the DNA fragments. Each DNA fragment binds to a primer on the flow cell, and bridge amplification occurs. In this process:
- The DNA strand bends over, forming a bridge on the surface.
- The bridge amplifies through polymerase chain reaction (PCR), creating multiple copies of the DNA fragment at the same location on the flow cell.
- This process results in the formation of dense clusters of identical DNA fragments, which enhance the signal during sequencing.
- Sequencing by Synthesis (SBS) The actual sequencing takes place in the Sequencing by Synthesis (SBS) process. Fluorescently labeled nucleotides (A, T, C, G) are added to the flow cell, and as each nucleotide is incorporated into the growing DNA strand, it emits a unique fluorescence signal corresponding to the base (A, T, C, or G). The process is as follows:
- Incorporation: The system adds one nucleotide at a time to the growing strand of DNA.
- Fluorescence detection: The emitted fluorescence from each incorporated nucleotide is captured by the sequencing system’s camera.
- Base calling: The sequence is determined by interpreting the fluorescence signals. Each signal corresponds to a specific nucleotide, allowing the system to accurately read the sequence of bases.
- After each cycle, the fluorescent tag is cleaved, and the next nucleotide is added. This cycle repeats until the entire fragment is sequenced.
- Data Analysis After sequencing, the raw data (fluorescent signals) are captured and processed using bioinformatics tools. The analysis process includes:
- Base calling: Converting the fluorescence signals into a sequence of nucleotides (A, T, C, G).
- Quality control: Ensuring the data is accurate and free from errors.
- Alignment: The sequences are aligned against a reference genome, or if no reference is available, de novo assembly is performed.
- Variant detection: Identifying genetic variants, such as SNPs (single nucleotide polymorphisms), insertions, deletions, and structural variations.
- Interpretation: The analyzed data are interpreted to identify potential biomarkers, gene functions, and pathways related to disease, development, or other biological processes.
Advantages of Illumina Sequencing
- High throughput: Millions of DNA fragments are sequenced simultaneously, which allows for massive data generation in a single run.
- High accuracy: The use of reversible terminators and real-time imaging ensures minimal sequencing errors.
- Cost-effective: Illumina sequencing is more affordable than traditional methods like Sanger sequencing, especially for large-scale projects.
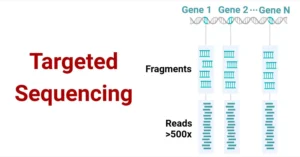
- Flexibility: Supports a wide range of applications from whole-genome sequencing to targeted resequencing, RNA sequencing, metagenomics, and more.
- Speed: Illumina platforms offer rapid sequencing, making them particularly useful for clinical diagnostics and time-sensitive research.
Limitations of Illumina Sequencing
- Short read length: Illumina sequencing typically produces short reads (100-300 base pairs), which can make assembling complex genomes or highly repetitive regions challenging.
- High initial investment: The cost of purchasing and maintaining Illumina sequencers can be expensive for small labs or institutions.
- Data management: The vast amount of data generated requires powerful computational tools and bioinformatics expertise for analysis.
- Overclustering: If too much DNA is loaded onto the flow cell, the resulting clusters can overlap, reducing the quality and accuracy of the sequencing data.
Applications of Illumina Sequencing
Illumina sequencing supports a wide range of applications in genomics and molecular biology:
- Whole-genome sequencing: Allows for the sequencing of entire genomes to explore genetic variations, mutations, and other genomic features.
- RNA sequencing (RNA-seq): Used to measure gene expression levels and analyze transcriptomes.
- Metagenomics: Sequencing environmental samples to study microbial communities and their functions.
- ChIP-seq (Chromatin Immunoprecipitation sequencing): Used to investigate protein-DNA interactions and histone modifications.
- Cancer research: Identifies mutations in cancer cells, helping in the identification of therapeutic targets and understanding tumorigenesis.
- Forensic analysis: Helps identify individuals or study relationships based on genetic material from crime scenes.
- Clinical diagnostics: Illumina sequencing is widely used in clinical settings for diagnosing genetic disorders, detecting pathogens, and monitoring disease progression.
Illumina sequencing has revolutionized the field of genomics by enabling high-throughput, accurate, and cost-effective sequencing. Its applications span a wide array of fields, from basic research to clinical diagnostics, making it a valuable tool in understanding genetics, diseases, and biological processes. However, challenges such as short read lengths and high initial costs need to be considered when deciding whether Illumina sequencing is the right choice for a given application.