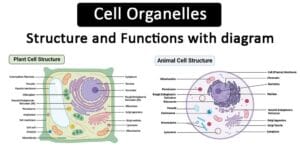
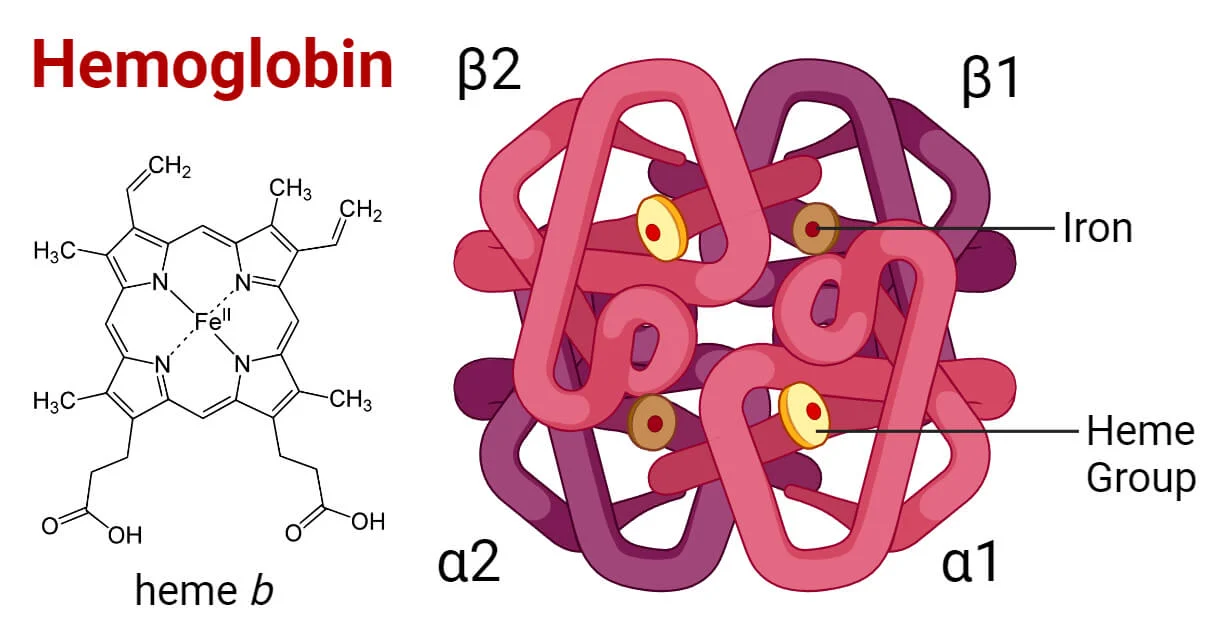
Structure of Hemoglobin:
Hemoglobin is composed of four protein molecules (globulin chains) bound together. These globulin chains are of two types: alpha and beta. In adults, hemoglobin has a tetramer structure consisting of two alpha and two beta chains. Each chain contains an iron atom, which binds to an oxygen molecule.
Types of Hemoglobin:
- Hemoglobin A (HbA): The most common type in adults, composed of two alpha and two beta globulin chains.
- Hemoglobin A2 (HbA2): Present in small amounts in adults, composed of two alpha and two delta globulin chains.
- Hemoglobin F (HbF): Predominant in fetal life, composed of two alpha and two gamma globulin chains.
Functions of Hemoglobin:
- Oxygen Transport: Hemoglobin binds with oxygen in the lungs and carries it to tissues and organs where it is needed for various metabolic processes.
- Carbon Dioxide Transport: Hemoglobin aids in transporting carbon dioxide, a waste product of metabolism, from tissues back to the lungs for exhalation.
- Buffering: Hemoglobin helps in maintaining the pH of blood by acting as a buffer, preventing excessive acidity or alkalinity.
Diseases Related to Hemoglobin:
- Anemia: A condition characterized by a decrease in the number of red blood cells or a deficiency of hemoglobin, leading to reduced oxygen-carrying capacity.
- Sickle Cell Disease: A genetic disorder where the structure of hemoglobin is altered, causing red blood cells to become rigid and assume a sickle shape.
- Thalassemia: An inherited blood disorder involving reduced production of hemoglobin and red blood cells.
- Polycythemia: A condition where there is an excess of red blood cells and hemoglobin in the blood, leading to increased blood viscosity.
Understanding the structure, types, functions, and associated diseases of hemoglobin is crucial for comprehending the vital role it plays in maintaining the body’s oxygen balance and overall health.
Structure of Hemoglobin:
Hemoglobin (Hgb) is a globular metalloprotein with a quaternary structure, and its detailed composition is as follows:
- Subunits and Molecular Weight:
- One hemoglobin molecule comprises four subunits, each with a polypeptide chain attached to a prosthetic heme group.
- Each subunit has a weight of approximately 16,000 Dalton, resulting in a total molecular weight of 64,000 Dalton for a hemoglobin molecule.
- Polypeptide Chains:
- In adults, the polypeptide chains consist of two types: alpha (α) and beta (β).
- Alpha chains (α1 and α2) contain 141 amino acids each, while beta chains (β1 and β2) contain 146 amino acids each.
- Adult hemoglobin has two alpha subunits and two beta subunits, forming two αβ dimers arranged with 2-fold axis symmetry.
- Fetal hemoglobin replaces beta subunits with gamma subunits (γ1 and γ2), while in some rare cases, beta subunits are replaced by delta subunits (δ1 and δ2).
- Heme Group:
- The heme group, a prosthetic group in hemoglobin, contains a ferrous ion (Fe+2) situated at the center of a porphyrin ring.
- The iron ion binds with nitrogen atoms of the porphyrin ring and is attached to the globin subunit, specifically with a histidine residue.
- Each Fe+2 ion in the heme group can bind to either one oxygen (O2) molecule or one carbon dioxide (CO2) molecule.
Understanding the detailed structure of hemoglobin, including its subunits, molecular weight, and the composition of the heme group, is essential for comprehending its crucial role in oxygen and carbon dioxide transport within the body.
Types of Hemoglobin:
Hemoglobin exists in various types, and the main ones are classified based on their non-alpha subunits:
- Hemoglobin A (HbA):
- Composition: It constitutes approximately 95 to 98% of total adult hemoglobin.
- Subunit Configuration: HbA consists of two alpha subunits and two beta subunits.
- Significance: It is the predominant and normal hemoglobin in adults, crucial for oxygen transport.
- Hemoglobin A2 (HbA2):
- Composition: HbA2 accounts for about 2 to 3% of total adult hemoglobin.
- Subunit Configuration: It contains two alpha subunits and two gamma subunits.
- Significance: HbA2 is a minor component of adult hemoglobin, contributing to its diversity.
- Hemoglobin F (HbF):
- Composition: Hemoglobin F is the hemoglobin found in fetuses and newborns, and it constitutes less than 1% in adults.
- Subunit Configuration: It consists of two alpha subunits and two delta subunits.
- Significance: HbF plays a crucial role in fetal development and is gradually replaced by HbA after birth.
Other Mutated Forms:
- Apart from the major types, there are mutated forms of hemoglobin, including:
- Hemoglobin E (HbE)
- Hemoglobin S (HbS)
- Hemoglobin C (HbC)
Understanding the different types of hemoglobin is essential for comprehending variations in its structure and function, as well as the implications of different forms in health and disease.
Functions of Hemoglobin:
Hemoglobin, a crucial component of red blood cells, performs several vital functions in the human body:
- Oxygen Transport:
- Mechanism: The iron (Fe+2) ion in the heme group binds with oxygen (O2), allowing one hemoglobin molecule to carry up to 4 oxygen molecules.
- Oxyhemoglobin: When oxygen is bound to hemoglobin, it forms oxyhemoglobin.
- Deoxyhemoglobin: Hemoglobin without bound oxygen is referred to as deoxyhemoglobin.
- Significance: Approximately 98% of oxygen in the blood is transported by oxyhemoglobin.
- Carbon Dioxide Transport:
- Mechanism: The heme group’s Fe+2 ion can also bind with carbon dioxide (CO2), enabling each hemoglobin molecule to carry up to 4 CO2 molecules.
- Carbaminohemoglobin: When CO2 is bound to hemoglobin, it forms carbaminohemoglobin.
- Significance: About 20 to 25% (average 23%) of carbon dioxide in the blood is transported by carbaminohemoglobin.
- Transport of Other Gases/Ions:
- Ligands: Hemoglobin can bind with various other substances, including carbon monoxide (CO), nitric oxide (NO), sulfur monoxide (SO), nitrite ion (NO-2), sulfides (S-2), etc.
- Carboxyhemoglobin: When hemoglobin binds with carbon monoxide, it forms carboxyhemoglobin. The affinity of hemoglobin for CO is more than 200 times that for oxygen.
- Regulation of Blood pH and Buffering:
- Mechanism: Hemoglobin molecules can bind to hydrogen ions, contributing to the maintenance of blood pH.
- Buffering Function: Hemoglobin acts as a buffer, helping to stabilize the pH of the blood.
Understanding these functions highlights the critical role hemoglobin plays in respiratory processes, ion transport, and pH regulation within the bloodstream.
Normal Hemoglobin Level:
The concentration of hemoglobin in the blood is expressed in grams per deciliter (g/dl), and the normal level can vary based on factors such as age, sex, and health status. Here is a general guide to normal hemoglobin levels in different age groups:
| Age of Person | Normal Hgb Level (g/dl) |
| Newborn | 14 to 24 |
| 2 weeks | 13 to 20 |
| 3 months | 9.5 to 14.5 |
| 6 months to 6 years | 10.5 to 14.0 |
| 6 years to 12 years | 11 to 16 |
| Adult male | 14 to 18 |
| Adult female | 12 to 16 |
It’s essential to note that these are general ranges, and individual variations may occur. Healthcare professionals use these reference values to assess and monitor an individual’s hemoglobin levels for health evaluation and potential medical intervention. Deviations from the normal range may indicate various health conditions that require further investigation. Regular monitoring and interpretation by healthcare providers are crucial for accurate health assessments.
Diseases Related to Hemoglobin:
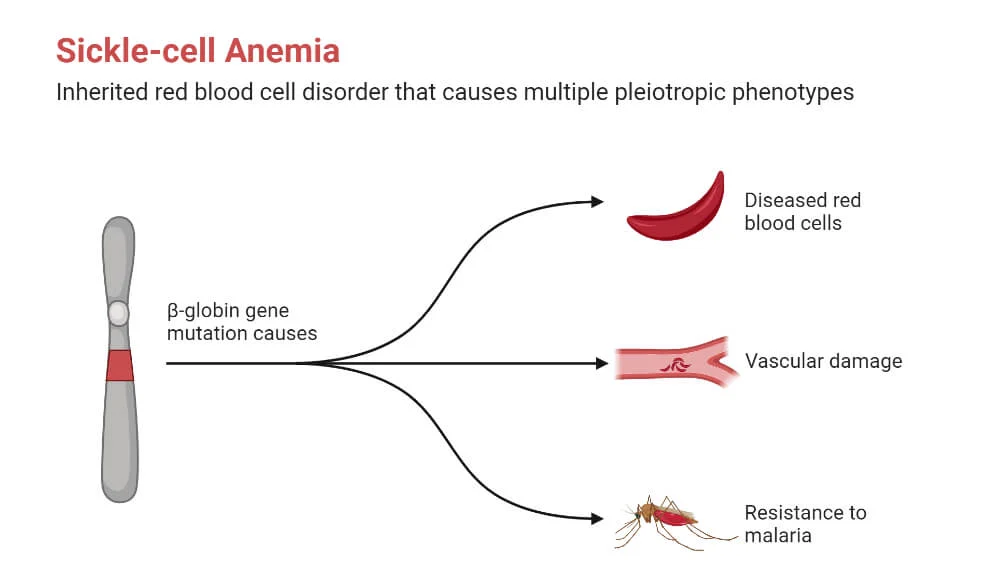
- Sickle Cell Disease:
- Description: Sickle Cell Disease is a genetic condition where the body produces abnormal hemoglobin, known as hemoglobin S, due to a mutation in the beta-globin gene HBB. This abnormal hemoglobin causes red blood cells to take on a sickle or crescent shape, leading to various health complications.
- Thalassemia:
- Description: Thalassemia is an inherited blood disorder characterized by a reduction in hemoglobin production. This reduction can result from the partial or complete absence of one or more globin subunits, leading to an imbalance in the synthesis of alpha and beta globin chains.
- Polycythemia:
- Description: Polycythemia is a condition marked by elevated levels of hemoglobin in the blood, causing an increase in red blood cell counts. This condition can result from various factors, including underlying health issues or excessive production of red blood cells by the bone marrow.
- Methemoglobinemia:
- Description: Methemoglobinemia is a disorder where the ability of hemoglobin to carry oxygen is reduced. This reduction occurs due to a change in iron from the ferrous (Fe+2) to the ferric (Fe+3) state. It can be acquired or inherited, and symptoms may include bluish discoloration of the skin and mucous membranes.
- Hemoglobinuria:
- Description: Hemoglobinuria is the presence of hemoglobin in the urine, indicating the breakdown of red blood cells. This condition may result from various factors, including hemolytic anemia or other disorders leading to the destruction of red blood cells.
- Hereditary Persistence of Fetal Hemoglobin (HPFH):
- Description: HPFH is a benign condition characterized by the continued presence of fetal hemoglobin (hemoglobin F) in adults in significant quantities. Unlike other hemoglobinopathies, HPFH generally does not cause significant health issues and is considered a benign variant.
These conditions can have diverse impacts on an individual’s health, ranging from mild to severe. Management and treatment approaches are often recommended based on the specific disorder, and regular medical monitoring is crucial for individuals with hemoglobin-related disorders.