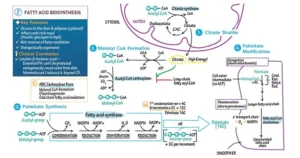
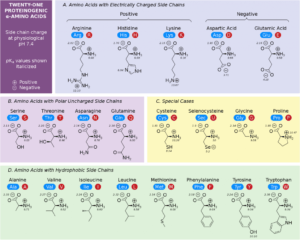
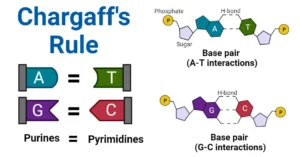
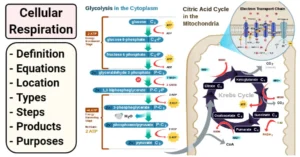
Biomedical Importance
Enzymes are indispensable biological catalysts that accelerate virtually every biochemical reaction in living organisms. Their extraordinary specificity and efficiency make them critical for metabolism, signal transduction, DNA replication, immune defense, and drug metabolism. Because enzyme activity changes in many diseases, enzymes and their isoforms also serve as important diagnostic and prognostic markers.
Enzymes Are Effective & Highly Specific Catalysts
Unlike nonbiological catalysts, enzymes function at mild temperatures and physiological pH, often increasing reaction rates by factors of millions. Their active sites bind substrates with remarkable specificity, minimizing unwanted side reactions and enabling precise metabolic control.
Enzyme Classification by Reaction Type & Mechanism
Enzymes are grouped into six major classes by the International Union of Biochemistry and Molecular Biology (IUBMB): oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. This classification reflects both the type of reaction catalyzed and the mechanism involved.
- Oxidoreductases catalyze oxidations and reductions.
- Transferases catalyze the transfer of groups such as methyl or glycosyl groups from a donor molecule to an acceptor molecule.
- Hydrolases catalyze the hydrolytic cleavage of C-C, C-O, C-N, P-O, and certain other bonds, including acid anhydride bonds.
- Lyases catalyze cleavage of C-C, C-O, C-N, and other bonds by elimination, leaving double bonds, and also add groups to double bonds.
- Isomerases catalyze geometric or structural changes within a single molecule.
- Ligases catalyze the joining together of two molecules, coupled to the hydrolysis of a pyrophosphoryl group in ATP or a similar nucleoside triphosphate.
Prosthetic Groups, Cofactors, & Coenzymes in Catalysis
- Prosthetic Groups are tightly bound nonprotein components integrated into an enzyme’s structure (e.g., flavin adenine dinucleotide in flavoproteins).
- Cofactors are usually inorganic ions (like Zn²⁺, Mg²⁺, or Fe²⁺) that associate reversibly with enzymes or substrates.
- Coenzymes are organic molecules, often derived from vitamins, that act as transient carriers of electrons, atoms, or functional groups during catalysis.
Many coenzymes, cofactors, and prosthetic groups originate from B-vitamins, explaining their essential nutritional role.

Catalysis Occurs at the Active Site
The active site is a three-dimensional cleft or pocket where the substrate binds. Its shape, polarity, and chemical groups create a microenvironment optimized for catalysis.
Enzymes Employ Multiple Mechanisms to Facilitate Catalysis
Enzymes accelerate reactions through several overlapping strategies:
- Catalysis by Proximity: Bringing substrates into close contact and correct orientation.
- Acid–Base Catalysis: Donating or accepting protons to stabilize intermediates.
- Catalysis by Strain: Distorting substrate bonds to resemble the transition state.
- Covalent Catalysis: Forming transient covalent enzyme–substrate intermediates.
Substrates Induce Conformational Changes
The “induced fit” model explains how enzymes adjust their conformation upon substrate binding, improving catalytic efficiency and specificity.
Illustrative Examples
- HIV Protease demonstrates acid–base catalysis, a mechanism exploited by antiretroviral drugs.
- Chymotrypsin (a digestive serine protease) exemplifies covalent catalysis via an acyl–enzyme intermediate.
- Fructose-2,6-Bisphosphatase also uses covalent catalysis to regulate carbohydrate metabolism.
Catalytic Residues Are Highly Conserved
Amino acids critical for catalysis (such as serine in chymotrypsin or histidines in many active sites) are strongly conserved across species, underlining their essential roles.
Isozymes: Distinct Enzyme Forms for the Same Reaction
Isozymes differ in amino acid sequence but catalyze the same reaction. They allow tissue-specific regulation and serve as powerful clinical biomarkers (e.g., lactate dehydrogenase isoenzymes).

Enzymes as Analytical Tools
Because enzymatic reactions are easy to monitor, enzyme activity forms the basis of many assays:
- Enzyme-Linked Immunoassays (ELISA): Use enzyme conjugates to detect antigens or antibodies.
- NAD(P)+-Dependent Dehydrogenases: Measured spectrophotometrically by NADH absorbance at 340 nm.
- Coupled Enzyme Assays: Use a secondary dehydrogenase to generate a measurable product.

Enzymes in Diagnosis
Leakage of normally intracellular enzymes into plasma signals tissue damage. For instance, measuring LDH isozyme patterns helps detect myocardial infarction. Similarly, enzyme analysis aids diagnosis of genetic diseases where enzyme deficiencies occur.

Recombinant DNA in Enzyme Research
Molecular biology has revolutionized the study of enzymes:
- Recombinant Fusion Proteins can be tagged and purified by affinity chromatography.
- Site-Directed Mutagenesis allows researchers to alter specific amino acids and probe catalytic mechanisms in detail.
Summary
- Enzymes are highly effective and extremely specific catalysts.
- Organic and inorganic prosthetic groups, cofactors, and coenzymes play important roles in catalysis. Coenzymes, many of which are derivatives of B vitamins, serve as “shuttles.”
- Catalytic mechanisms employed by enzymes include the introduction of strain, approximation of reactants, acid-base catalysis, and covalent catalysis.
- Aminoacyl residues that participate in catalysis are highly conserved among all classes of a given enzyme activity.
- Substrates and enzymes induce mutual conformational changes in one another that facilitate substrate recognition and catalysis.
- The catalytic activity of enzymes reveals their presence, facilitates their detection, and provides the basis for enzyme-linked immunoassays.
- Many enzymes can be assayed spectrophotometrically by coupling them to an NAD(P)+-dependent dehydrogenase.
- Assay of plasma enzymes aids diagnosis and prognosis. For example, a myocardial infarction elevates serum levels of lactate dehydrogenase isozyme I1.
- Restriction endonucleases facilitate diagnosis of genetic diseases by revealing restriction fragment length polymorphisms.
- Site-directed mutagenesis, used to change residues suspected of being important in catalysis or substrate binding, provides insights into the mechanisms of enzyme action.
- Recombinant fusion proteins such as His-tagged or GST fusion enzymes are readily purified by affinity chromatography.