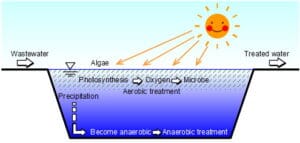
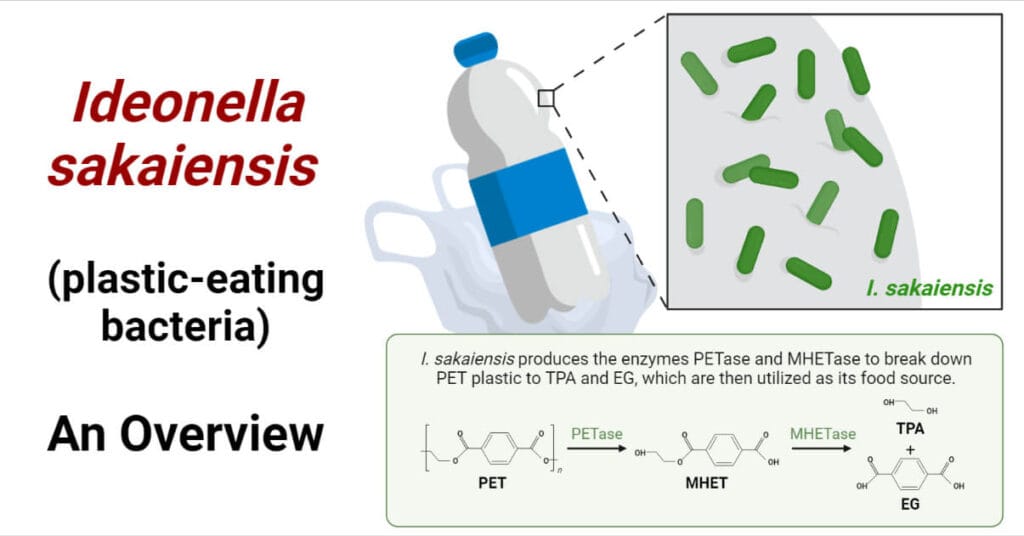
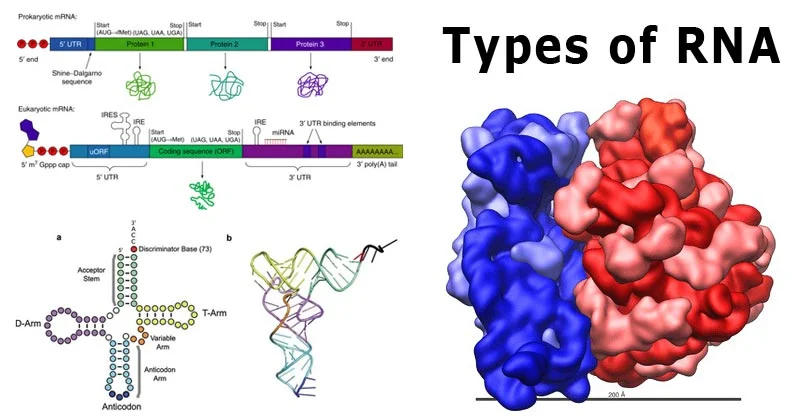
The biokinetic properties of a xenobiotic refer to how the substance is absorbed, distributed, metabolized, and excreted (ADME) in a biological system. These properties determine the xenobiotic’s fate, toxicity, and potential therapeutic or harmful effects. Understanding biokinetics is essential for risk assessment, drug development, and environmental safety evaluations.

1. Absorption
Absorption describes the process by which a xenobiotic enters the systemic circulation from its site of administration or exposure.
- Routes of Absorption:
- Oral: Passive diffusion through the gastrointestinal tract.
- Inhalation: Rapid absorption through alveoli in the lungs.
- Dermal: Diffusion through skin layers.
- Injection: Direct entry into the bloodstream or tissues.
- Factors Influencing Absorption:
- Chemical Properties: Lipophilicity, molecular size, and polarity.
- pH and pKa: Affect ionization and membrane permeability.
- Biological Barriers: Intestinal epithelium, skin, or alveolar membranes.
2. Distribution
Distribution is the transport of the xenobiotic from the bloodstream to tissues and organs.
- Key Determinants:
- Plasma Protein Binding: Xenobiotics bind to plasma proteins like albumin, affecting their free concentration.
- Tissue Affinity: Lipophilic xenobiotics accumulate in fatty tissues, while hydrophilic ones are distributed in aqueous compartments.
- Blood Flow: High-perfusion organs like the liver, kidneys, and brain receive xenobiotics rapidly.
- Barriers: Structures like the blood-brain barrier restrict the entry of certain xenobiotics.
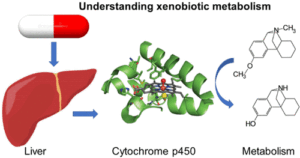
3. Metabolism
Metabolism involves the biotransformation of the xenobiotic to more water-soluble forms for excretion, often in the liver.
- Phases of Metabolism:
- Phase I (Functionalization):
- Involves oxidation, reduction, or hydrolysis.
- Enzymes like cytochrome P450s play a key role.
- Phase II (Conjugation):
- Xenobiotics are conjugated with glucuronic acid, sulfate, or glutathione to increase solubility.
- Phase I (Functionalization):
- Metabolites:
- Active Metabolites: Retain or enhance biological activity.
- Toxic Metabolites: May cause cellular or organ damage (e.g., acetaminophen’s toxic metabolite).
4. Excretion
Excretion removes the xenobiotic or its metabolites from the body.
- Routes of Excretion:
- Renal: Primary route for hydrophilic xenobiotics.
- Hepatic: Lipophilic compounds excreted in bile and feces.
- Pulmonary: Volatile xenobiotics excreted via exhalation.
- Other Routes: Sweat, saliva, and breast milk.
- Factors Influencing Excretion:
- Molecular size, polarity, and ionization.
- Renal function and hepatic enzyme activity.
5. Biokinetic Parameters
Quantitative measures are used to describe the biokinetic behavior of xenobiotics:
- Half-Life (t1/2): Time required to reduce the xenobiotic concentration by half.
- Volume of Distribution (Vd): Apparent volume in which the xenobiotic is distributed.
- Clearance (CL): Rate of xenobiotic elimination from the body.
- Bioavailability (F): Fraction of the administered dose that reaches systemic circulation.
6. Special Considerations
- Bioaccumulation: Lipophilic xenobiotics may accumulate in fat tissues over time.
- Species Variability: Different species metabolize xenobiotics differently due to enzymatic variations.
- Age and Health: Metabolic rates and excretory efficiency differ in neonates, the elderly, and individuals with liver or kidney diseases.
Biokinetic properties govern the behavior and impact of xenobiotics in biological systems. A thorough understanding of ADME processes and biokinetic parameters is critical for designing safe drugs, managing toxic exposures, and assessing environmental risks.