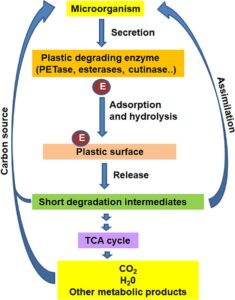
BioBleaching typically refers to the use of biological agents for bleaching processes. This concept is particularly significant in industries like paper, textile, and wastewater treatment, where it aims to replace or reduce the use of harmful chemical bleaches such as chlorine, which are environmentally damaging.

Applications and Mechanisms:
- Pulp and Paper Industry:
- BioBleaching involves enzymes like laccases, xylanases, and lignin peroxidases.
- These enzymes break down lignin (a major component that gives color to pulp), making the paper-whitening process more eco-friendly.
- Textile Industry:
- Enzymatic treatments such as cellulases and oxidoreductases are used for fabric whitening without damaging fibers or releasing toxic byproducts.
- Wastewater Treatment:
- BioBleaching agents can detoxify dye-laden industrial effluents, making water reuse feasible.
Advantages:
- Reduces reliance on toxic chemicals like chlorine and hypochlorite.
- Lowers energy consumption as the biological process often occurs at milder conditions.
- Minimizes harmful by-products like dioxins and furans.
- Enhances sustainability in industrial processes.
Challenges:
- Enzyme production and stability at industrial scales can be costly.
- The efficiency of enzymes in harsh industrial environments needs optimization.
- Compatibility with existing industrial processes can require adaptation.
Biobleaching of Ores
Biobleaching of valuable metals from ores, also known as bioleaching or biomining, is an eco-friendly and cost-effective method for extracting metals using biological processes. This approach leverages microorganisms to catalyze the breakdown of metal-containing minerals, facilitating the release of metals into solution. Here’s a detailed overview of the organisms, mechanisms, and processes involved in the biobleaching of valuable metals:
1. Key Microorganisms Involved
Microorganisms involved in bioleaching are specialized extremophiles that thrive in acidic and metal-rich environments. They can oxidize sulfide minerals or reduce insoluble metal compounds, facilitating the solubilization of metals. Commonly used microbes include:
A. Bacteria
- Acidophilic bacteria:
- Acidithiobacillus ferrooxidans: Oxidizes ferrous iron (Fe²⁺) to ferric iron (Fe³⁺) and sulfur to sulfate, aiding in sulfide mineral breakdown.
- Acidithiobacillus thiooxidans: Specializes in oxidizing sulfur compounds, creating acidic environments to enhance metal solubilization.
- Leptospirillum ferrooxidans: Oxidizes iron, generating ferric iron as an oxidizing agent.
- Heterotrophic bacteria:
- Pseudomonas spp.: Contributes to complexation and mobilization of metals via organic acids and biosurfactants.
B. Fungi
- Aspergillus niger and Penicillium spp.: Produce organic acids (e.g., citric acid) that solubilize metals through chelation.
C. Archaea
- Sulfolobus metallicus and Ferroplasma acidarmanus: Thermophilic and acidophilic archaea that operate in extreme environments, especially for high-temperature ore leaching.
2. Mechanisms of Biobleaching
The biobleaching process involves biological and chemical reactions that lead to the solubilization of metals from ores. The main mechanisms are:
A. Direct Mechanism
Microorganisms directly attack the mineral surface by enzymatic and biochemical activity, dissolving the metal. For example:
- Sulfide minerals like pyrite (FeS₂) are oxidized directly by microbes.
Reaction Example:
FeS2 + 7O2 + 2H2O→ 2Fe2+ + 4SO4 2-+ 4H+
B. Indirect Mechanism
Microbes generate oxidizing agents such as ferric iron (Fe³⁺) or protons (H⁺), which then react chemically with the ore to extract metals. This mechanism is particularly important in the oxidation of sulfide ores.
Reaction Example:
- Microbial oxidation of ferrous iron to ferric iron:
4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O - Chemical attack on sulfide ore by ferric iron:
MS + 2Fe3+ → M2+ + S + 2Fe2+
Here, M represents the metal being leached (e.g., copper or zinc).
C. Organic Acid Production
Fungi and heterotrophic bacteria produce organic acids (e.g., citric acid, oxalic acid), which chelate and solubilize metals from ores.
Reaction Example:
M2CO3 + H2C6H5O7 → 2M2+ + CO2 + C6H6O72−
3. Biobleaching Process
The process of biobleaching involves several steps, with variations depending on the type of ore, the target metal, and the microorganisms used.
A. Types of Ores Suitable for Biobleaching
- Sulphide Ores: Copper (chalcopyrite, covellite), zinc (sphalerite), gold (arsenopyrite).
- Oxide Ores: Uranium (pitchblende), manganese.
- Polymetallic Ores: Containing multiple valuable metals like copper, cobalt, nickel.
B. Steps in the Biobleaching Process
- Ore Preparation:
- Crushing and grinding of ores to increase surface area for microbial action.
- Inoculation:
- Introduction of microbial cultures (bacteria, fungi, or archaea) into the system.
- Leaching Process:
- Heap Leaching: Ores are piled into heaps, and leachate (acidic solution containing microbes) is percolated through the heap.
- Tank Leaching: Ores are placed in tanks with controlled microbial and environmental conditions.
- In-Situ Leaching: Microbial solutions are injected directly into ore deposits underground.
- Metal Recovery:
- Metals dissolved in the leachate are recovered via methods such as precipitation, solvent extraction, or electrowinning.
- Effluent Treatment:
- The acidic and metal-laden effluent is neutralized and treated before discharge.
4. Examples of Metals Extracted via Biobleaching
- Copper: Leached from chalcopyrite using A. ferrooxidans.
- Gold: Extracted from refractory ores where microbes oxidize sulfide minerals to release encapsulated gold.
- Uranium: Bioleached using A. thiooxidans and A. ferrooxidans from low-grade ores.
- Zinc and Nickel: Extracted from sphalerite and pentlandite, respectively.
5. Advantages of Biobleaching
- Environmentally Friendly: Reduces the need for toxic chemicals like cyanide or sulfuric acid.
- Cost-Effective: Lower operational costs compared to traditional smelting or roasting techniques.
- Efficient for Low-Grade Ores: Makes it viable to extract metals from ores with low metal concentrations.
- Reduced Energy Consumption: Operates at ambient temperatures and pressures.
6. Limitations and Challenges
- Slow Process: Microbial leaching is time-intensive compared to chemical leaching.
- Acidic Effluents: Requires proper handling and neutralization to prevent environmental damage.
- Microbial Sensitivity: Microorganisms are sensitive to fluctuations in temperature, pH, and toxic metal concentrations.
- Scale-Up Issues: Laboratory successes may face challenges during large-scale implementation.
7. Applications
- Mining Industry: Recovery of base metals like copper, zinc, and nickel.
- Gold Processing: Treatment of refractory gold ores.
- Environmental Cleanup: Remediation of mine tailings and metal-polluted sites.
Biobleaching represents a promising approach to sustainable metal extraction, especially as demand for valuable metals increases while ore quality decreases. Research into optimizing microbial consortia and improving reaction kinetics continues to advance this field.