Biomedical Importance of Amino Acids
Amino acids are the basic building blocks of proteins, which perform structural, enzymatic, transport, and signaling functions in all living organisms. Beyond protein synthesis, amino acids serve as precursors for hormones, neurotransmitters, nucleotides, and other biomolecules. For example, tryptophan is the precursor for serotonin and melatonin, while tyrosine gives rise to dopamine, epinephrine, and thyroid hormones. Because of these roles, amino acids are central to metabolism, immune responses, tissue repair, and clinical nutrition.
Properties of Amino Acids
The Genetic Code Specifies 20 L–Amino Acids
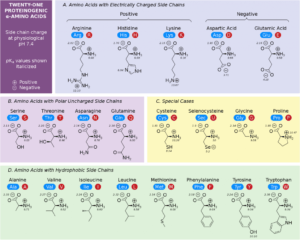
The universal genetic code encodes 20 standard amino acids that are incorporated into proteins during translation. These amino acids differ mainly in their side chains (R-groups), which confer unique chemical properties.
Only L–Amino Acids Occur in Proteins
Although amino acids can exist as D- and L-enantiomers (optical isomers), ribosomal protein synthesis incorporates only L-amino acids. This stereospecificity is critical for proper protein folding and biological function.
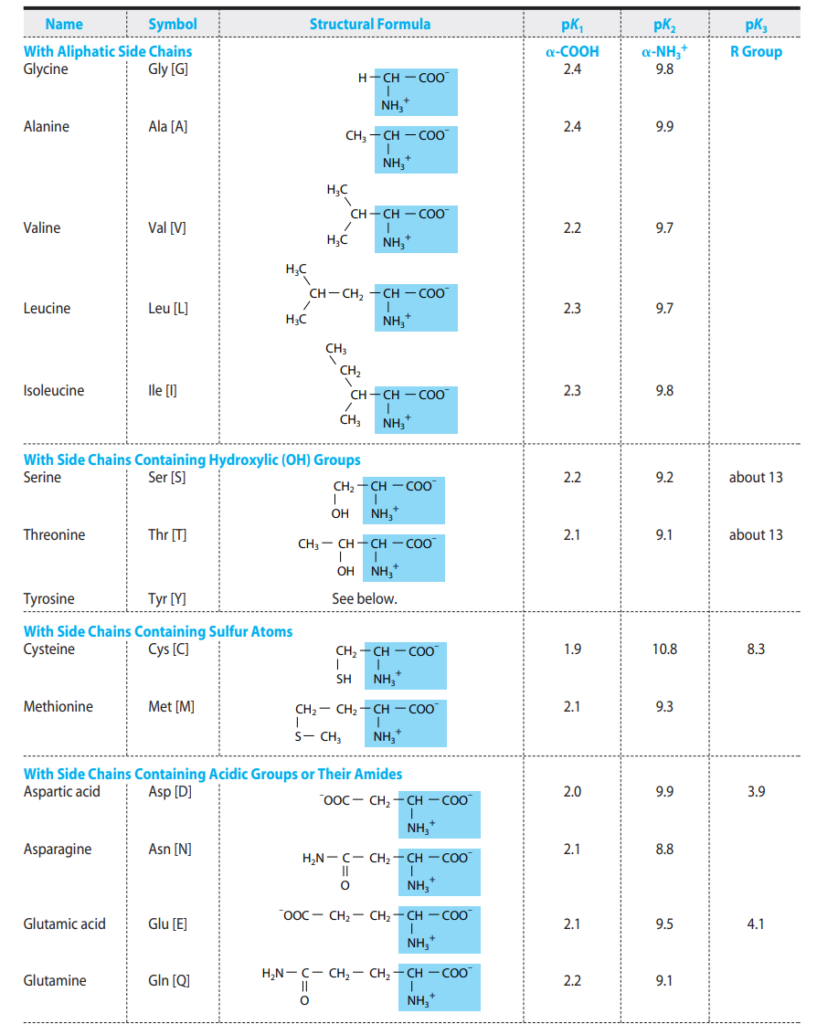
Amino Acids May Have Positive, Negative, or Zero Net Charge
At physiological pH (~7.4), the amino and carboxyl groups are ionized, and side chains may also carry charges. For example, lysine and arginine are positively charged, glutamate and aspartate are negatively charged, and many nonpolar residues (e.g., leucine) are neutral. This charge diversity influences solubility, interactions, and enzymatic activity.
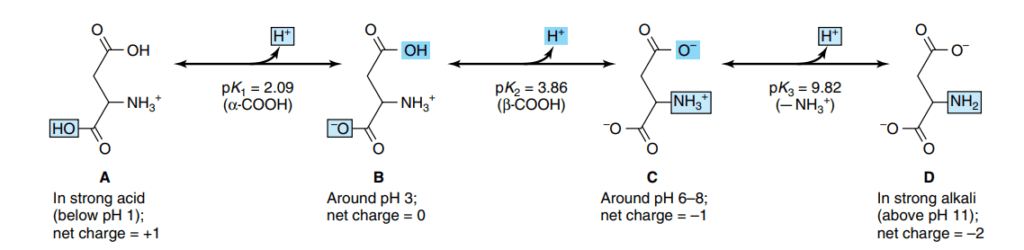
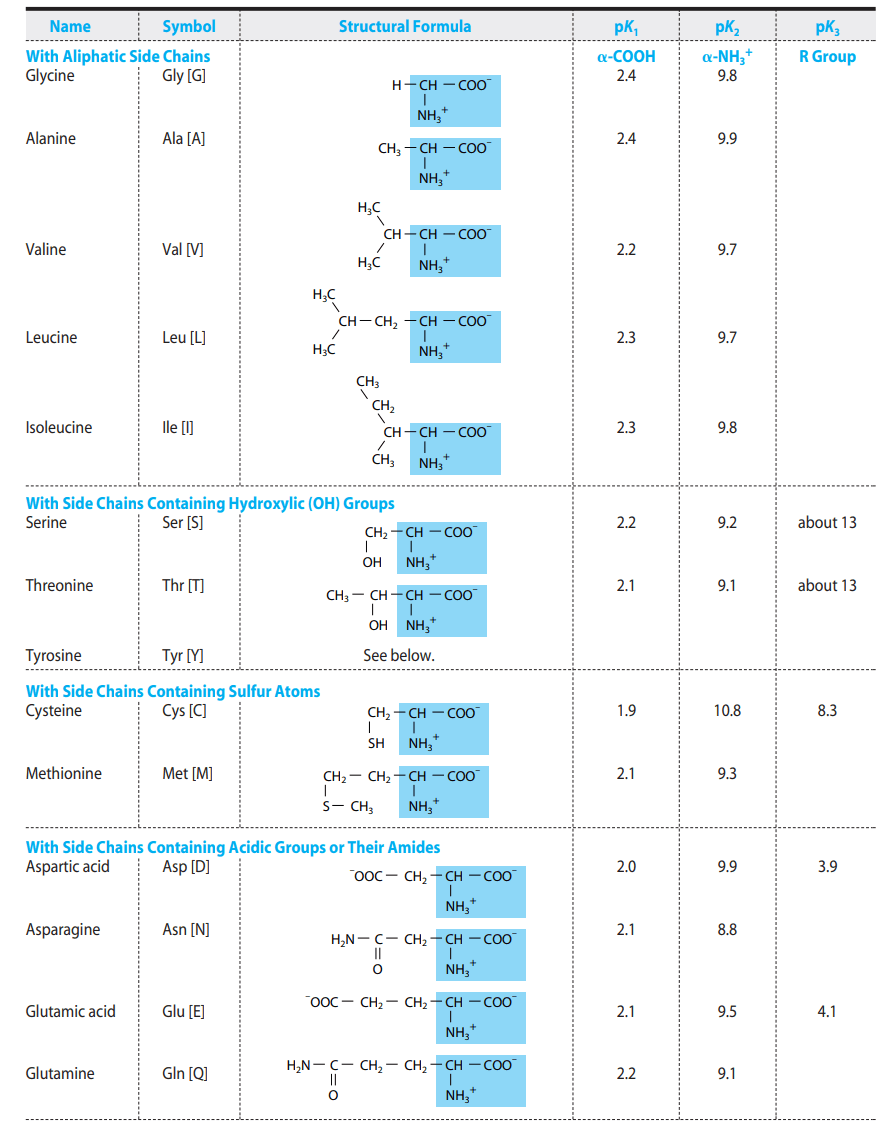
pKa Values Express the Strengths of Weak Acids
Each ionizable group of an amino acid has a characteristic pKa. The pKa reflects how readily the group donates or accepts protons. The α-carboxyl group typically has a pKa of ~2, and the α-amino group ~9–10, while side chains vary widely.
At Its Isoelectric pH (pI), an Amino Acid Bears No Net Charge
The isoelectric point (pI) is the pH at which the sum of positive and negative charges equals zero. At this pH, amino acids are least soluble and migrate minimally in an electric field—principles exploited in techniques like isoelectric focusing.
pKa Values Vary With the Environment
The microenvironment within a protein can shift the pKa of an amino acid. For instance, a carboxyl group buried in a hydrophobic core may exhibit a much higher pKa than in water. Such shifts affect protein stability and enzymatic catalysis.
The Solubility and Melting Points of Amino Acids Reflect Their Ionic Character
Because amino acids are zwitterions at neutral pH (bearing both positive and negative charges), most are highly soluble in water and have relatively high melting points compared with neutral organic molecules.
Functional Groups Dictate the Chemical Reactions of Amino Acids
The side chains determine chemical reactivity. For example, cysteine’s sulfhydryl group forms disulfide bonds, serine’s hydroxyl group can be phosphorylated, and histidine’s imidazole group often participates in acid–base catalysis. These reactions underlie the diverse functions of proteins.
Amino Acids and Protein Structure
Amino Acid Sequence Determines Primary Structure
The linear sequence of amino acids (primary structure) dictates how a protein folds into its higher-order structures (secondary, tertiary, quaternary). Even a single amino acid substitution can alter function dramatically, as in sickle-cell anemia.
Peptide Structures Are Easy to Draw
In peptide diagrams, amino acids are linked via peptide bonds in a straightforward N-to-C direction. Standard conventions help biochemists visualize and predict properties quickly.
Some Peptides Contain Unusual Amino Acids
While proteins are built from the 20 standard amino acids, some peptides include modified residues such as hydroxyproline (in collagen), γ-carboxyglutamate (in blood-clotting factors), or selenocysteine and pyrrolysine (coded by special mechanisms).
The Peptide Bond Has Partial Double-Bond Character
The peptide bond between the carboxyl group of one amino acid and the amino group of the next is planar and rigid due to resonance, giving it partial double-bond character. This restricts rotation and shapes protein backbones.
Noncovalent Forces Constrain Peptide Conformations
Hydrogen bonds, hydrophobic interactions, van der Waals forces, and ionic interactions collectively stabilize secondary and tertiary structures. These noncovalent forces allow proteins to adopt functional conformations.
Analysis of Amino Acid Content of Biological Materials
Determining amino acid composition is essential in research, nutrition, and clinical diagnostics. Standard methods include:
- Hydrolysis of proteins to release free amino acids.
- Chromatographic separation (HPLC, ion-exchange chromatography).
- Detection and quantification using ninhydrin, fluorescence, or mass spectrometry.
These analyses reveal protein purity, post-translational modifications, or metabolic disorders involving amino acid imbalances.
Summary
- Occurrence in Nature:
Both D-amino acids and non-α-amino acids exist in nature, but only L-α-amino acids are incorporated into proteins. - Functional Groups:
All amino acids contain at least two weakly acidic groups — an amino group (–NH₃⁺) and a carboxyl group (–COOH).
Many also have additional functional groups such as –OH, –SH, guanidino, or imidazole, which contribute to their chemical reactivity. - pKa and pI:
The pKa values of an amino acid’s ionizable groups determine its net charge at a given pH.
The isoelectric point (pI) is the pH at which the amino acid has no net charge and therefore does not migrate in an electric field. - Peptide Bond Formation:
The most important biochemical reaction of amino acids is the formation of peptide bonds, which link amino acids to form peptides and proteins. - Role of R-Groups:
The R-groups (side chains) of amino acids define their unique biochemical functions.
Based on R-group properties, amino acids are classified as basic, acidic, aromatic, aliphatic, or sulfur-containing. - Peptide Nomenclature and Structure:
Peptides are named according to the number of amino acid residues they contain and as derivatives of the carboxyl-terminal residue.
The primary structure of a peptide is its amino acid sequence, starting from the amino-terminal residue. - Peptide Bond Geometry:
The partial double-bond character of the peptide bond makes the four atoms around it coplanar and restricts rotation, thereby limiting possible peptide conformations.